How to Calculate Plans in Xrd Drawings
10-ray Diffraction
- Page ID
- 315
The construction of a simple powder diffractometer was first described by Hull in 1917one which was before long after the discovery of X-rays by Wilhelm Conrad Röntgen in 1895ii. Diffractometer measures the angles at which 10-rays get reflected and thus get the structural information they contains. Present resolution of this technique get meaning comeback and information technology is widely used every bit a tool to analyze the phase information and solve crystal structures of solid-state materials.
Introduction
Since the wavelength of X-rays is similar to the altitude between crystal layers, incident X-rays will be diffracted, interacting with sure crystalline layers and diffraction patterns containing of import structural data well-nigh the crystal tin exist obtained. The diffraction pattern is considered the fingerprint of the crystal because each crystal structures produce unique diffraction patterns and every phase in a mixture produces its diffraction design independently. We can employ grinded bulk sample into fine powders, which are typical under ten µm,ii as samples in powder X-ray Diffraction (XRD). Different single crystal 10-ray diffraction (X-ray Crystallography) technique, the sample will distribute evenly at every possible orientation and powder XRD collects one-dimensional information, which is a diagram of diffracted beam intensity vs. Bragg angle θ, rather than three-dimensional data.
Theoretical consideration
In this section, let us accept a wait at the theoretical basis of powder X-ray diffraction technique. (e.yard. lattice structures and how X-rays interacts with crystal structures)
Unit of measurement cells
"Crystals are built upwards of regular arrangements of atoms in three dimensions; these arrangements can exist represented by a repeat unit or motif called the unit cell."2 In crystallography, all the crystal unit cells tin be classied into 230 space groups. Some bones knowledge nigh crystallography is necessary for a well understanding of powder XRD technique. In crystallography, the basic possible classifications are: six crystal families, 7 crystal systems, five centering position, 14 Bravais lattices and 32 crystal classes.
Based on the angles and the length of the axes sides, unit cell can be divided into half dozen crystal families, which are cubic, tetragonal, hexagonal, orthorhombic, monoclinic and triclinic. As the hexagonal family tin can have ii different appearances, we can separate it into two systems which are trigonal lattice and hexagonal lattice. That is how the seven crystal systems generate. If forget the shape of lattice and just consider the atoms' positions, we tin can separate the lattices into primitive lattices and not-archaic ones. A primitive lattice (also defined as simple) is the lattice with the smallest possible atomic coordination number2, e.g.wheneight atoms lie in the 8 corners. And all the other lattices are called non-primitive lattice. Based on the three-dimension position of the atoms in the unit of measurement cell, we can divided the non-primitive lattice into 3 types: face centered (F), side centered (C), body centered (I) and based centered(R).
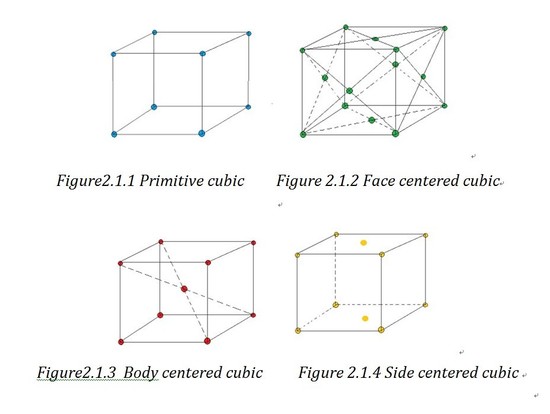
Bravais lattice is a "combination of lattice blazon and crystal systems"i.And you can find a chart of examples of all the 14 Bravais lattice in exterior link.
32 crystal classes refer to 32 crystallographic point group classfied by the possible symmetric operations, which are rotation, reflection and inversion. Yous may wonder why only 32 possible point grouping. The answer is crystallographic restriction, which means crystal system tin can simply have five kinds of rotation axises: 1-fold, 2-fold, three-fold, four-fold and 6-fold. To exist simplify, only the permissible rotation axises allow unit cells grow uniformly without any openings among them.
230 space groups are combinations of 14 Bravais lattice and 32 crystal classes. Those space groups are generated from translations of related Bravais lattice and glide plane and/or scew axis of relative crystal classes. They are represented by Hermann-Mauguin. For example, the NO.62 infinite grouping, Pnma is derived from D2h crystal class. P indicated it is primitive structure and n, m, a stand for a diagonal glide plane, a mirror aeroplane and a axial glide planes. The infinite group belongs to orthorhombic crystal family.
Miller Indices and Reciprocal Lattice
Miller indices and reciprocal lattice are essential to understanding the geometry of lattice planes and X-ray diffraction technique, because they are widely used to index the planes and orientations in crystallography and allow data handling in a uncomplicated and mathematical method. To assign the Miller indices (h,k,l) to a certain set of parallel planes which are defined every bit a plane family , kickoff nosotros need to find the first aeroplane side by side to the plane, passing through the origin. Then nosotros can observe the iii intersection of this plane on the unit cell vectors, a, b, c. "The Miller indices would be the reciprocals of the fractional intersections."1 Why we desire the reciprocal of fraction instead of the fraction directly? To get the respond, we need get to understand what reciprocal lattice is.
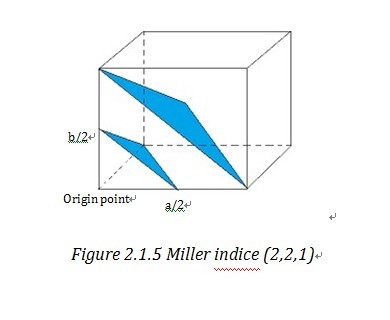
"Geometrically, the planes can be specified past ii quantities: (i) their orientation in the crystal and (two) their d-spacing." 4 (d-spacing is the interplanar distance) This allows u.s. to use a vector d*, which is normal to the planes and whose length is inverse to d-spacing, i.due east. d*=K/d 4 to stand for a certain family of planes. d* is called reciprocal lattice vector and similarly in 3 dimension system, reciprocal lattice vector d* hkl correspond (h,grand,l). And the end point of reciprocal lattice vector form a grid or lattice- reciprocal lattice unit jail cell four . Reciprocal lattice cell vector a*, b*, c* is reciprocal form of direct unit jail cell vector a, b, c. Then it is easy to find out that d* hkl =ha*+kb*+lc*. By take the reciprocal number of the intercepts of Miller indices, those two notation systems are very consistent and straightforward in indexing the crystal lattice.
Bragg's Law
Bragg' s law is the theoretical footing of 10-ray diffractometer. Let us consider the crystalline equally built up in planes. As shown in the diagram, X-ray beam shines into the planes and is reflected by different planes. The axle reflected by the lower airplane will travel an extra altitude (shown in Figure two.ii.one in red)than that reflected by the upper i,which is 2dsinθ. If that distance equals nλ(n is an integer), we volition go effective interference, which corresponds to the bright contrast in diffraction design. So the Bragg equation equally shown below defines the position of the existence of constructive diffraction at different orders.
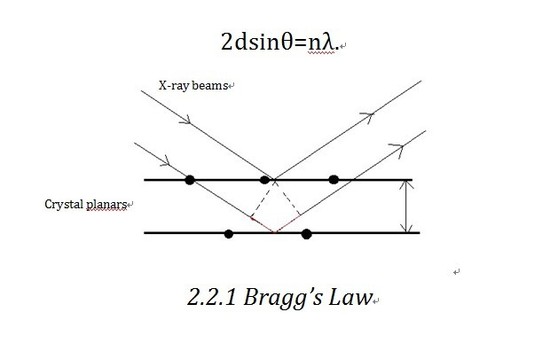
D-spacing, which is the inter plane distance d in Bragg's equation, is decided by the lattice parameter a, b, c, equally shown beneath.

Then subsequently finding out d-spacing from detected Bragg'south angle, we can effigy out the lattice parameter which contains vital structural information. Also nosotros can reconstruct the unknown structure by figuring out all the possible d-spacing. Powder XRD can define the stage contained in a mixture on the ground of separating and recognizing characteristic diffraction pattern.
Structure factor
The sample of pulverization X-ray diffraction volition distribute evenly at every possible orientation, and then after diffracted, the diffraction blueprint appears every bit circles with aforementioned center point instead of dots in single crystal diffraction patterns. The circles in the diffraction patterns with smaller radius correspond to smaller h, thou, l. In sure types of unit of measurement cells, not all the lattice planes will have their diffraction observed, which is commonly chosen systematic absence, because the diffracted axle may happen to be out of phase past 180°and the overall intensity would be aught. Structure factor Fhkl can make up one's mind the systematic absences and intensity.Systematic absences ascend when F=0, so no diffraction will be observed. For example:
For a fcc crystal, Fhkl=f{1+eastwardπ i (h+fifty)+eπ i (k+l)+eastwardπ i (h+m)}. When h, k, l are all odd or all even, F=4f. For the other situation, F=0 and thus diffraction intensity volition also be zero. Structure factor is important in the structure determination step because it helps empathize the Miller indices and intensities of diffraction peaks. The other common rules for reflection to be observed are listed every bit follows:
| Lattice type | Rule for reflection to be observed |
|---|---|
| Primitive, P | None |
| Body centered, I | hkl: h+k+l=2n |
| Confront centered, F | hkl: h, k, 50 either all odd or all fifty-fifty |
| Side centered, C | hkl: h+thousand=2n |
| Rhombohedral, R | hkl: -h+k+50=3n or (h-one thousand+50=3n) |
Instrumentation
Pulverisation 10-ray diffractometer consists of three components: X-ray source, sample holder and detector.
Source
Possible X-ray sources are X-ray tube, Synchrotron radiation and cyclotron radiation. X-ray tube equipped with filter is usually used in laboratory diffractometer. Synchrotron radiation is a brighter source and as a consequence can increase the resolution.

The cathode part of X-ray tube generated electrons nether electrical current. Electrons travel from cathode to anode through a high dispatch voltage, typically thirty~150kV. In this process, most of the free energy is released as oestrus and X-ray simply account for approximately i% of total free energy. The X-ray tube needs lasing cooling water to protect information technology from over heat while working. After Ten rays hitting the anode(red function in the schematic), the anode generates feature 10-rays, which comes from the process of excited electrons falling down to lower electron shell and stand for to the energy difference between electron shells. In Bruker D8 diffractometer, the anode is made of Cu, so the 10-ray souce is Cu-Ka1 and Cu-Ka2. K ways the electrons falls to K shell from higher shells. α means the excited electrons lies in Fifty shell, one shell college than One thousand shell. If the excited electron comes from G shell which is two electron beat higher, and so what we have is defined as Mβ. The divergence betwixt Cu-Thousanda1 and Cu-Ka2 is that they come from different subshell, Cu-Ka1 corresponds to 2p2/3 to 1s beat while Cu-Ka2 corresponds to 2p1/2 to 1s crush.
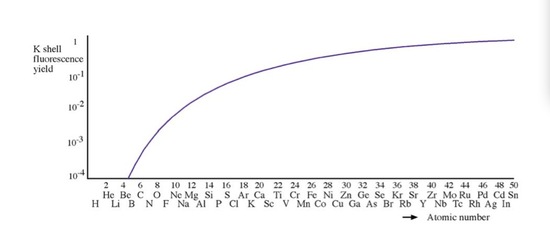
In a reflection geometry instrumentation, Ten-ray tube commonly contains a side window made of Exist to permit the generated X-rays to emit at the demanding angle. The reason that Be is used as a X-ray window, is that the fluorescence yield (ration between feature X ray and Auger emission) of Be is close to aught, and then it can make sure the X-ray source is monochromatic and does not contain introduced chracteristic X-rays from other metals.
Sample Holder
There are many holder options to holder all kinds of samples and run into people's requirement. Usually, evenly grinded sample powder is dissolved in organic solvent such as acetone or pressed into a apparently on a drinking glass slide to make sure the sampel is flat. The sample holder has a printing ring to fix the slide. At depression angles, the signal to noise ratio tin be relatively larger and we can use a zero background sample holder to avoid that. It is commonly made of single crystal silicon.
Detector
Photographic flick serves every bit detector in earlier methods, Debye-Scherrer camera and Guinier camera methods2. The film is placed around the sample as a circle and records the diffracted X-ray beams. The positions of diffraction lines stand for to Bragg angle. And Photographic film tin record both the reflected and transmitted X-ray beams. Nowadays, people tend to just select the reflected beam and use radiation counter as detector. Compared to pic, scintillationcounter tin measure diffraction intensities and Bragg angles more accurately. And it is very convenient utilize computer to analyze data.To prevent the 10-ray beam from going though the sample, we need more powder sample.
Application
Phase Assay
X-ray diffractometer is most widely used in the phase assay because compare to other characterization method, XRD gives a fast and reliable measurement(measurement time is determined by the pace size, angle range and the number of 2d per step) and easy sample preperation(well grinded pulverization). What people do after get their raw data is opening the data in a XRD data handling software (JADE, WinXPOW, ect.) and compare the raw data with the standard blueprint in ICDD database. In many cases, the database may not incorporate the pattern of a specific compound you are working on, and then you can easily generate a calculated design based on a crystal informtion file or the space group and lattice parameters. The diffraction blueprint of a well-prepared sample should be very reliable (all the peaks should match the peaks in the reference pattern) and contains much information. When the sample has impurities, every kinds of substance will generate their ain blueprint independently and allows the states to analysize seperately and aid people control and optimize the reactions. Other factor that can influence the pattern may be the X-ray souce, sample crystallinity (smaller crystallinity can broaden the peaks), ect.
XRD technique is also capable for quantitative analysis of mixtures. The XRD data will not give the quantitative imformation considering the intensity is not directly related to weight percantage. However, we can brand a serie of controlled samples with different weight percent of the impurity. Then XRD is performed on each of these sample and we can become a linear scale curve which is the intensity ratio vs. weight percentage. The weight percentage in the sample can be determined based on the curve. If all the atomic and crystalline data are known, we tin can also carry qualitative assay with Rietveld method. A square least approximation method will be applied to modify all the parameters so that the difference between experimental point and the fitted design can be decreased to a least amount. During this process, the scale factor can likewise be determined. Rietveld method is widely used in samples containing more than one impurities.
Structure conclusion
Powder X-ray diffraction can not only be used to analyze phase data,but can also be used to determine the structure of unknown substances. However, since in powder XRD we tin only get ane-dimensional information rather than three-dimensional data, resolution in pulverization XRD is much lower than that from unmarried crystal method and data refinement procedure is more than sophisticated."If representative single crystal method are available, then single crystal diffraction is the preferred method."i
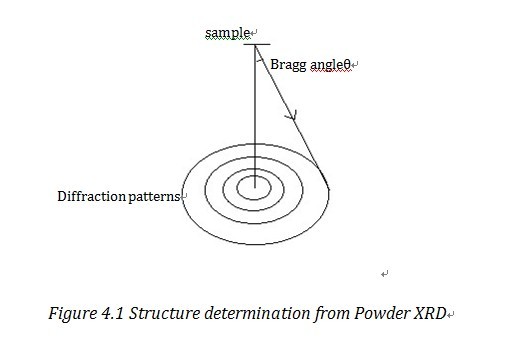
Equally shown in Effigy 4.ane, i Bragg Ring corresponds to a certain Miller airplane. With the detected Bragg angle and equation two.i, nosotros tin figure out the lattice parameters. The common method to manage information is directly method, Patterson method and Fourier method2. We can also use the foursquare least approximation method, i.east. Rietveld method to refine the data, to refine the data and information technology has increased the resolution a lot.
Common single crystal methods are Laue method, four-circle diffractometer and rotating-crystal method.
Reference
- W. I. F. David, K. Shankland, L. B. McCusker, Ch. Baerlocher; Structure Determination from Pulverization Diffraction Data; Oxford; New York : Oxford University Press, 2002
- Anthony R. Due west; Basic Solid State Chemistry, 2nd Edition; New York : John Wiley & Sons, c1999
- Transimission Electron Microscope, Book 1, David B. Williams, C. Barry Carter Springer,2009
- The Basics of Crystallography and Diffraction, Second Edition,Christopher Hammond;Oxford Science Publications, 2001
Problems
- Describe the lattice planes (ane,ane,one) (0,1,2) and (1,0,ane) in a cubic lattice.
- If we use Cu αradiation as Ten-ray source, and the first order Bragg diffraction peak is found at the semi-bending 35。,calculate the d-spacing of the crystal.
- Ten-rays with wavelength ane.54 A are reflected from the (2,1,1) planes of a cubic crystal. The d-spacing is found to be 5.12A. Calculate the crystal parameter.
- Show that for a body centered cubic lattice, reflection can be observed only when h+l+1000=2n
- If you lot need to conduct powder XRD on an air sensitive crystal, choose a proper sample holder you need on line.
Source: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Instrumental_Analysis/Diffraction_Scattering_Techniques/X-ray_Diffraction
0 Response to "How to Calculate Plans in Xrd Drawings"
Postar um comentário